2023-02-28
News:
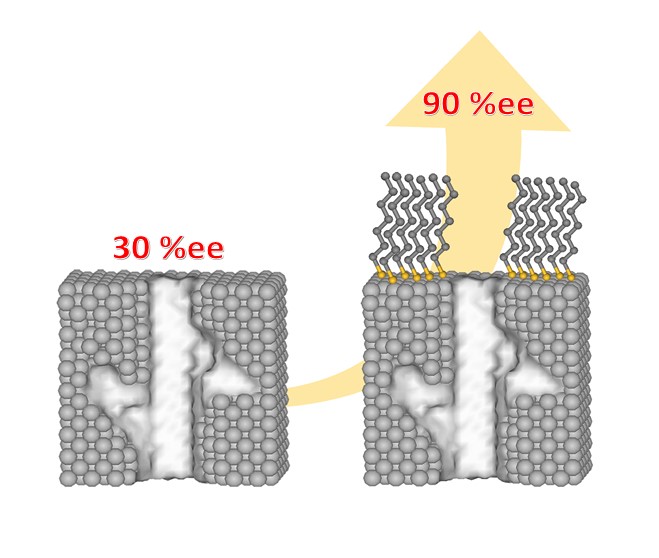
Conglomerate formation, where enantiomers within a racemic mixture self-segregate upon crystallization, is an advantageous property for obtaining chirally pure crystals and allows large-scale chiral resolution. However, the prevalence of conglomerates is low and difficult to predict. In this report, we describe our attempts to engineer conglomerates from racemate-forming compounds by integrating them into a conglomerate-forming matrix. In this regard, we found that Ni(II) and Fe(II) form molecular alloys with Zn(II) in [MxZn(1−x)(bpy)3](PF6)2 (where bpy = 2,2′-bipyridyl). Powder X-ray Diffraction (PXRD) and Energy-Dispersive X-ray spectroscopy (EDX) evidenced conglomerate crystallization with Ni(II) concentrations up to about 25%, while it was observed only for much lower concentrations of Fe(II). This can be attributed to the ability of [Ni(bpy)3](PF6)2 to access a metastable conglomerate phase, while no such phase has been detected in [Fe(bpy)3](PF6)2. Furthermore, the chiral phase appears to be favored in fast-growing precipitates, while the racemic phase is favored in slow re-crystallizations for both Ni(II) and Fe(II) molecular alloys. X-ray natural circular dichroism (XNCD) measurements on [Ni0.13Zn0.87(bpy)3](PF6)2 demonstrate the chirality of the nickel molecules within the zinc molecular matrix.
U. Serdan, L. Robin, M. Marchivie, M. Gonidec, P. Rosa, E. Duverger-Nédellec, E. Pouget, P. Sainctavit, M.-A. Arrio, A. Juhin, A. Rogalev, F. Wilhelm and E. A. Hillard Chemistry. 5(1) (2023) 255-268

fig :Molecular alloy of conglomerate [Ni@Zn(bpy)3](PF6)2
U. Serdan, L. Robin, M. Marchivie, M. Gonidec, P. Rosa, E. Duverger-Nédellec, E. Pouget, P. Sainctavit, M.-A. Arrio, A. Juhin, A. Rogalev, F. Wilhelm and E. A. Hillard Chemistry. 5(1) (2023) 255-268

Laboratory :
ICMCB