Chirality Expression from Hierarchical Foldamer-Mesoscopic Helical Silica Frameworks
2025-06-03
Chirality Expression from Hierarchical Foldamer-Mesoscopic Helical Silica Frameworks.
We elucidate how chiral structures of vastly different sizes can interact and subsequently transfer their chiral information.
Materials Chemistry Frontiers, 2025,
9, 1501 - 1510
https://doi.org/10.1039/D4QM01140F
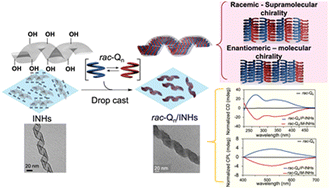
Laboratory: CBMN-IECB_ISM
Elucidating chirality transfer in liquid crystals of viruses
2024-05-30
How does the propagation of chirality across various length scales take place?
It unfolds within the intricate playground of virus self-assemblies – used as model system of chiral particles – to form helical liquid crystals. These chiral liquid crystals exhibit a fascinating duality: simple in structure yet complex in behavior. This work explores the physical mechanisms governing chirality transfer for two distinct virus mutants, M13 and Y21M. Despite their high structural similarity, these viruses exhibit cholesteric liquid crystalline phase phases with opposite handedness. This seemingly apparent paradox stems from the subtle interplay between steric repulsion, chiral supramolecular virus deformations, and electrostatic forces.
For the stiff Y21M virus strain, the local electrostatic interactions, which are highly sensitive to both ionic content and the detailed atomic symmetries of the capsid, play a crucial role. In this case, the molecular chirality originates primarily from the subtle helical distribution of surface charges around the symmetry axes of the capsid.
In contrast, the chirality of the M13 virus arises from weak, fluctuation-induced suprahelical deformations of its backbone. This chirality transfer and the resulting helical phase are chiefly driven by steric interactions occuring at the supramolecular length scale of the viruses themselves.
Overall, this study provides a quantitative description of chirality transfer across different length scales. It reveals the complex interplay of competing chiral interactions with opposite signs, governing the emergence of chiral structures and helical morphologies.
Laboratory: CRPP
seminar of Prof. Yoshihiko Togawa (Osaka Metropolitan Univeresity) : A role of chirality: Generation and transfer of spin and phonon angular momenta
2024-07-05
We discuss a role of chirality in materials, being inspired by recent studies on chirality-induced selectivity of spin and phonon angular momenta with chiral materials. A comprehensive understanding of these nontrivial phenomena will clarify the interplay between structural and dynamical chirality.
when ? 05/07, 14h-15h
where ? IECB
Laboratory: CBMN
Bulk electrosynthesis of patchy particles with highly controlled asymmetric features
2024-01-16
Asymmetric modification of particles with various patches of different composition and size at predefined positions is an important challenge in contemporary surface chemistry, as such particles have numerous potential applications, ranging from materials science and (photo)catalysis to self-assembly and drug delivery. However, approaches allowing the synthesis of this kind of complex objects in the bulk of a solution in a straightforward way are currently lacking. In this context, bipolar electrochemistry (BE) is a powerful technique for the asymmetric modification of conducting objects. Herein, this approach is used for the highly controlled modification of particles with different metal patches, generated at specific locations of isotropic objects. The synthesis is carried out in the bulk of the solution and leads to predefined patterns of increasing complexity,
including even a specific chiral arrangement of the patches.
Laboratory: ISM
Modulation of circularly polarized luminescence by swelling of microgels functionalized with enantiopure [Ru(bpy)3]2+ luminophores
2023-12-20
Chemoresponsive microgels functionalized with enantiomeric D- or
K-[Ru(bpy)3] 2+ showed tunable chiroptical properties upon swelling
and shrinking. The tuning is triggered by a modulation of the local
mobility of [Ru(bpy)3]2+ upon addition of fructose, controlling inter-
actions and distances between [Ru(bpy)3]2+ and phenylboronic acid.

 DOI: 10.1039/D3CC04391F
DOI: 10.1039/D3CC04391FLaboratory: CBMN/ISM/Univ. Genève/Kumamoto Univ/Tohoku Univ.
Chirality Related Properties in Helicene and Tetrathiafulvalene based Materials
2023-12-22
Who ?
Dr. Narcis AVARVARI du Laboratoire MOLTECH Anjou (Université d'Angers).
Where ? salle de conférences de l'ISM (3e étage)
When ? 22 dec. 2023, 10h30-11h30
Keywords: CISS effect, eMChA effect, Helicene, Conductivity, TTF
Abstract and references on :
https://www.ism.u-bordeaux.fr/actualites/dr-narcis-avarvari
Laboratory: ISM
Deadline sur PEPR LUMA
2023-09-15
Le PEPR LUMA invite les chercheurs travaillant dans les axes de recherche sur la chiralité listés sur :
https://www.pepr-luma.fr/axes/chiralite/
de déposer des lettres d'intention (<3 pages) avant le 15 sept. 2023 afin de construire lors de workshops en visioconférence (dates prévisionnelles 27-28 sept. 2023) des consortia (typiquement 10 équipe de recherche travaillant ensemble) qui répondront aux AMI-appel à manifestation d'intérêts- auprès de l'ANR-PEPR-LUMA en automne 2023. Le template de la lettre d'intention et les dates clefs sont consultables sur :
https://www.pepr-luma.fr/appels/appel-a-manifestations-dinteret-recherche-thematique/
NB : les AMI sont des financements sur 5 ans n'incluant pas de l'équipement >50 keuros.
Laboratory: chiralité
Chirogenesis in Solid State and Spontaneous Resolution
2023-04-18
Chirality is a property of asymmetry resulting, for an object, from the
non-superposition of its image in a mirror. The notion of symmetry
breaking, inherent in the organization of matter, the formation of new
structural edifices, and, more fundamentally, weak interactions, is omnipresent. From the physics of elementary particles to molecules of
the living world and functional organisms, to climatic phenomena inducing
vortices of forces, chirality often plays a crucial role. It is also a
conception of geometry exploited in design fields and man-made constructions for its functionality and uniqueness.
In this chapter, we will focus on the chirality observed in solid state
matter, that is, chirality based on the solid state organization of atoms or
molecules. While there can be an important overlap with inorganic chiral
nanostructures or nanoparticles for which there are a number of reviews,
the solid state matters treated in this chapter can include crystals as well
as amorphous solids of both organic and inorganic molecules. As we
will discuss below, the study of the chirality of solid materials has
mainly been focused on asymmetric ordered and periodic structures.
When atoms are considered as a repeating unit, chiral crystals of achiral
molecules can be classified as 3D asymmetric periodic structures. Chiral
crystal faces of centric crystals and chiral 2D patterns of achiral molecules
can be classified as 2D asymmetric periodic structures. Individual
helical polymeric chains, chiral carbon nanotubes, and nanoparticles can
be classified as 1D asymmetric periodic structures. We should also mention
that chiral quasicrystals do not have mirror symmetry or translational
symmetry, but have rotational symmetry, showing beautiful chiral
ordered structures. We will also describe how chirality can be enhanced
by the 2D or 3D organization of building components of solid materials.
We will close with a discussion of spectroscopic methods to characterize
chiral objects and assemblies.
Reiko Oda, Peizhao Liu, Elizabeth Hillard, Patrick Rosa, Sylvain Nlate, Yutaka Okazaki, Emilie Pouget, Yann Battie and Thierry Buffeteau
https://doi.org/10.1142/9789811259227_0006

Laboratory: CBMN, ISM, ICMCB, Université de Lorraine, Kyoto University
Disentangling Excimer Emission from Chiral Induction in Nanoscale Helical Silica Scaffolds Bearing Achiral Chromophores
2023-04-18
The synthesis and characterization of diketopyrrolopyrroles and perylenemonoimidodiesters linked to a substituted benzoic acid in the
ortho,
meta, and
para positions, are reported. Grafting of these dyes on the surface of chiral silica nanohelices is used to probe how the morphology of the platform at the mesoscopic level affects the induction of chiroptical properties onto achiral molecular chromophores. The grafted structures are weakly (diketopyrrolopyrroles) or strongly (perylenemonoimidodiesters) emissive, exhibiting both locally-excited state emission and a broad, structureless emission assigned to excimers. The dissymmetry factors obtained using circular dichroism highlight optimized supramolecular organization between the chromophores for enhancing the chiroptical properties of the system. In the
ortho- derivatives, poor organization due to steric hindrance is reflected in a low density of chromophores on walls of the silica-nanostructures (<0.1 vs. >0.3 and up to 0.6 molecules/nm
2 for the
ortho and
meta or
para derivatives, respectively) and lower
gabs values than in the other derivatives (
gabs<2×10
−5 vs 6×10
−5 for the
ortho and
para derivatives, respectively). The
para derivatives presented a better organization and increased values of
gabs. All grafted chromophores evidence varying degrees of excimer emission which was not found to directly correlate to their grafting density.
Maria João Álvaro-Martins, José Garcés-Garcés, Antoine Scalabre, Peizhao Liu, Fernando Fernández-Lázaro, Ángela Sastre-Santos, Dario M. Bassani, Reiko Oda
ChemPhysChem 2023, 24, e202200573

Laboratory: CBMN, ISM, Universidad Miguel Hernández
Chiral shaped perovskite nanocrystals growth inside hollow silica nanoribbons
2023-04-18
Helical perovskite nanocrystals (H-PNCs) were prepared using nanometric silica helical ribbons as platforms for the in-situ growth of the crystals using supersaturated recrystallization method. The H-PNCs grow inside nanometric helical porous silica, their handedness are determined by the handedness of porous silica templates. They show strong induced circular dichroism (CD) and induced circularly polarized luminescence (CPL) signals, with very high dissymetric g-factors. Right-handed and left-handed PNCs show respectively positive and negative CD and CPL signals, with dissymmetric g-factor (abs and lum) of ~ +/- 2*10-2. Simulations based on the boundary element method demonstrate that the circular dichroism comes from the chiral shape of H-PNCs.
Peizhao Liu, Yann Battie, Takaki Kimura, Yutaka Okazaki, Piyanan Pranee, Hao Wang, Emilie Pouget, Sylvain Nlate, Takashi Sagawa, and Reiko Oda
https://doi.org/10.1021/acs.nanolett.2c04823

Laboratory: CBMN
The 2nd day meeting of kinoa
2023-03-15
The Research Federation "Chirality in Nouvelle Aquitaine" (Ki-NOA) is organizing its 2nd day of scientific meetings and exchanges on the 25th of April at the IECB (2 rue Robert Escarpit, Pessac). This event will take place from 8h50-17h15, and the program is available at
https://kinoa.cnrs.fr/2nd-kinoa-meeting/
Registration mandatory before the 7th of April on the framaform sent by email.
Laboratory: all labs
The kinoa tour
2023-02-22
Nous avons eu 10 propositions de visite de labo sur le campus bordelais pour échanger autour de la chiralité. Ces visites se sont tenues entre décembre 2022 et février 2023, avec des échanges scientifiques très constructifs. Merci à tous ceux qui ont donné de leur temps et curiosité 🙂
Laboratory: all labs
2nd Kinoa Meeting-2023
Laboratory:
Félicitation à Alexander et toute l’équipe NSYSA de l’ISM
2023-03-06
Alexander Kuhn, membre très actif de la fédération kinoa, professeur à l’École nationale supérieure de matériaux, d’agroalimentaire et de chimie et chercheur à l’Institut des Sciences Moléculaires a recu l'une des 24 médailles d'argent du CNRS octroyée en 2023. Félicitation de tous à l'équipe NSYSA (NanoSystèmes Analytiques)
https://www.cnrs.fr/fr/personne/alexander-kuhn
https://nsysa.ism-bordeaux.cnrs.fr/staff/permanent/22-staff/288-contact-alexander-kuhn-2.html

Laboratory: ISM
Crystal Engineering of Conglomerates: Dilution of Racemate-Forming Fe(II) and Ni(II) Congeners into Conglomerate-Forming [Zn(bpy)3](PF6)2
2023-02-28
Conglomerate formation, where enantiomers within a racemic mixture self-segregate upon crystallization, is an advantageous property for obtaining chirally pure crystals and allows large-scale chiral resolution. However, the prevalence of conglomerates is low and difficult to predict. In this report, we describe our attempts to engineer conglomerates from racemate-forming compounds by integrating them into a conglomerate-forming matrix. In this regard, we found that Ni(II) and Fe(II) form molecular alloys with Zn(II) in [M
xZn
(1−x)(bpy)
3](PF
6)
2 (where bpy = 2,2′-bipyridyl). Powder X-ray Diffraction (PXRD) and Energy-Dispersive X-ray spectroscopy (EDX) evidenced conglomerate crystallization with Ni(II) concentrations up to about 25%, while it was observed only for much lower concentrations of Fe(II). This can be attributed to the ability of [Ni(bpy)
3](PF
6)
2 to access a metastable conglomerate phase, while no such phase has been detected in [Fe(bpy)
3](PF
6)
2. Furthermore, the chiral phase appears to be favored in fast-growing precipitates, while the racemic phase is favored in slow re-crystallizations for both Ni(II) and Fe(II) molecular alloys. X-ray natural circular dichroism (XNCD) measurements on [Ni
0.13Zn
0.87(bpy)
3](PF
6)
2 demonstrate the chirality of the nickel molecules within the zinc molecular matrix.
U. Serdan, L. Robin, M. Marchivie, M. Gonidec, P. Rosa, E. Duverger-Nédellec, E. Pouget, P. Sainctavit, M.-A. Arrio, A. Juhin, A. Rogalev, F. Wilhelm and E. A. Hillard
Chemistry. 5(1) (2023) 255-268
 fig :Molecular alloy of conglomerate [Ni@Zn(bpy)3](PF6)2
fig :Molecular alloy of conglomerate [Ni@Zn(bpy)3](PF6)2Laboratory: ICMCB
Autonomous Chiral Microswimmers with Self‐mixing Capabilities for Highly Efficient Enantioselective Synthesis
2022-12-15
The development of chiral catalysts plays a very important role in various areas of chemical science. Heterogeneous catalysts have the general advantage of allowing a more straightforward separation from the products. One specific case of heterogeneous catalysis is electrocatalysis, being potentially a green chemistry approach. However, a typical drawback is that the redox conversion of molecules occurs only at the electrode/electrolyte interface, and not in the bulk of the electrolyte. The second limitation is that the electrodes have to be physically connected to a power supply to induce the desired reactions. To circumvent these problems, we propose here a complementary approach by replacing macroscopic electrodes with an ensemble of self-propelled redox active microswimmers. They move autonomously in solution while transforming simultaneously a prochiral starting compound into a specific enantiomer with a very high enantiomeric excess, accompanied by a significantly increased production rate of the favorite enantiomer.
S. Arnaboldi, G. Salinas, G. Bonetti, P. Garrigue, R. Cirilli, T. Benincori, A. Kuhn
Angew.Chem.Int.Ed. 61 (2022) e202209098
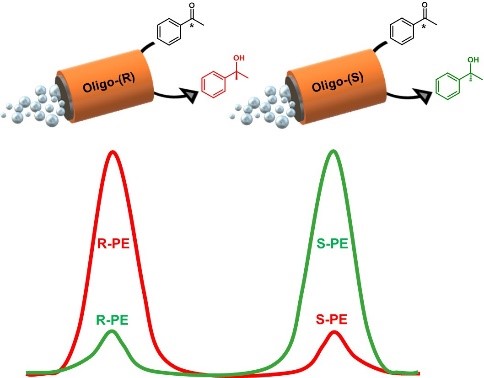
Laboratory: ISM
Electrochemiluminescent enantioselective detection with chiral-imprinted mesoporous metal surfaces
2022-12-15
Chiral-imprinted mesoporous Pt-Ir alloy surfaces were combined in a synergetic way with electrochemiluminescence (ECL) to detect the two enantiomers of phenylalanine (PA) as a model compound, acting simultaneously as a chiral target and as a co-reactant to generate significant differences in ECL signals. The chiral features of the metal surfaces are converted into an enantioselective electrogeneration of the excited state of the [Ru(bpy)3]2+ dye, which in fine produces the differentiating light emission with up to 20-fold differences in intensity for the two enantiomers. These findings open up the possibility of developing new ECL-based bioassays and microscopy of chiral environments.
S. Butcha, J. Yu, Z. Pasom, B. Goudeau, C. Wattanakit, N. Sojic, A. Kuhn
ChemComm 58 (2022) 10707-10710

Laboratory: ISM
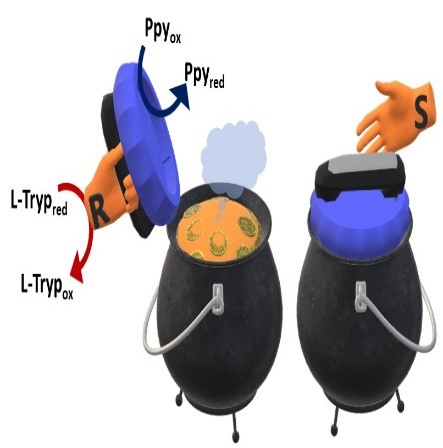
Bipolar electrochemical rotors for the direct transduction of molecular chiral information
2022-12-15
Efficient monitoring of chiral information of bioactive compounds has gained considerable attention, due to their involvement in different biochemical processes. In this work, we propose a novel dynamic system for the easy and straightforward recognition of chiral redox active molecules and its possible use for the efficient measurement of enantiomeric excess in solution. The approach is based on the synergy between the localized enantioselective oxidation of only one of the two antipodes of a chiral molecule and the produced charge-compensating asymmetric proton flux along a bipolar electrode. The resulting clockwise or anticlockwise rotation is triggered only when the probe with the right chirality is present in solution. The angle of rotation shows a linear correlation with the analyte concentration, enabling the quantification of enantiomeric ratios in mixtures where the two antipodes are present in solution. This device was successfully used to simultaneously measure different ratios of the enantiomers of 3,4-dihydroxyphenylalanine and tryptophan. The versatility of the proposed approach opens up the possibility to use such a dynamic system as a straightforward (bio)analytical tool for the qualitative and quantitative discrimination of different redox-active chiral probes.
S. Arnaboldi, G. Salinas, G. Bonetti, R. Cirilli, T. Benincori, A. Kuhn
Biosens. Bioelectron. 218 (2022) 114740

Laboratory: ISM
Wireless electromechanical enantio-responsive valves
2022-12-15
Microfluidic valves based on chemically responsive materials have gained considerable attention in recent years. Herein a wireless enantio-responsive valve triggered by bipolar electrochemistry combined with chiral recognition is reported. A conducting polymer actuator functionalized with the enantiomers of an inherently chiral oligomer was used as bipolar valve to cover a tube loaded with a dye, and immersed in a solution containing chiral analytes. When an electric field is applied, the designed actuator shows a reversible cantilever-type deflection, allowing the release of the dye from the reservoir. The tube can be opened and closed by simply switching the polarity of the system. Qualitative results show the successful release of the colorant, driven by chirality and redox reactions occurring at the bipolar valve. The device works well even in the presence of chemically different chiral analytes in the same solution. These systems open up new possibilities in the field of microfluidics, including also controlled drug delivery applications.
G. Salinas, F. Malacarne, G. Bonetti, R. Cirilli, T. Benincori, S. Arnaboldi, A. Kuhn
Chirality (2022) in press
Laboratory: ISM
Self-assembled monolayer protection of chiral-imprinted mesoporous platinum electrodes for highly enantioselective synthesis
2022-12-15
In modern chemistry, chiral (electro)catalysis is a powerful strategy to produce enantiomerically pure compounds (EPC). However, it still struggles with uncontrollable stereochemistry due to side reactions, eventually producing a racemic mixture. To overcome this important challenge, a well-controlled design of chiral catalyst materials is mandatory to produce enantiomers with acceptable purity. In this context, we propose the synergetic combination of two strategies, namely the elaboration of mesoporous Pt films, imprinted with chiral recognition sites, together with the spatially controlled formation of a self-assembled monolayer. Chiral imprinted metals have been previously suggested as electrode materials for enantioselective recognition, separation and synthesis. However, the outermost surface of such electrodes is lacking chiral information and thus leads to unspecific reactions. Functionalising selectively this part of the electrode with a monolayer of organosulfur ligands allows an almost total suppression of undesired side reactions and thus leads to a boost of enantiomeric excess to values of over 90% when using these surfaces in the frame of enantioselective electrosynthesis. In addition, this strategy also decreases the total reaction time by one order of magnitude. The study therefore opens up promising perspectives for the development of heterogeneous enantioselective electrocatalysis strategies.
S. Butcha, V. Lapeyre, C. Wattanakit, A. Kuhn
Chem.Sci., 13, 2022, 2339
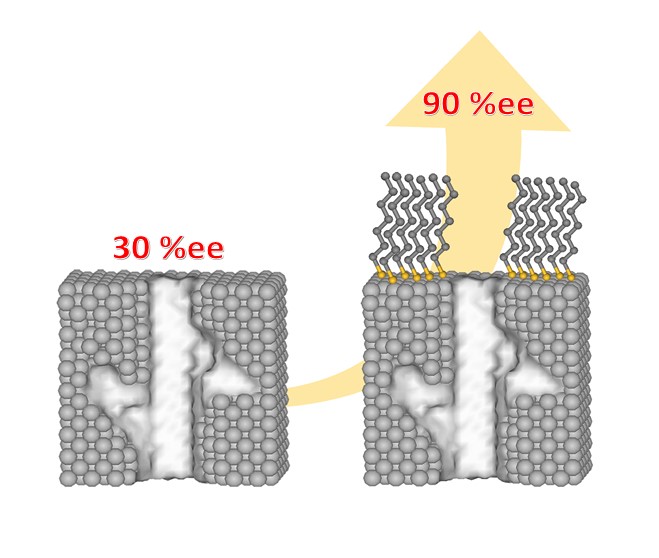
Laboratory: ISM
Two Prizes during JACC2022 conference
2022-10-06
Two prizes were delivered during JACC2022 :
- Paola Matozzo (Univ. Rennes-PhD student at ISCR) for the poster prize for her presentation on ''Exciton coupling chirality in helicene-porphyrin conjugates''
- Nicolas Bruni (Univ. Bordeaux-PhD student at LOMA) for the young talk prize for his presentation on ''Directed morphogenesis of rewritable chiral liquid crystal supramolecular structures by chiral light''


Laboratory: JACC2022
GDR PES “Photo-Electro-Stimulation”
2022-10-20
The ISM will host the next symposium of the GDR PES "Photo-Electro-Stimulation". The goal of this GDR is to put together photochemists, spectroscopists and electrochemists to discuss about the interplay between these fields.
The forthcoming symposium will take place from Monday 7th to Wednesday 9th of November at the ENSCBP school in Pessac.
Here is the website to register:
https://jpes.sciencesconf.org/
You should create an account prior to register in case you do not have already one.
Registration is free but compulsory !Laboratory: ISM
INSCRIPTION TO JACC 2022
2022-06-02
Journées André Collet de la Chiralité
- October 4-7 (2022) in a wonderfull place in Biarritz
- deadline for oral abstract submission :
June 15th July 15-2022
- deadline for poster abstract submission : August 31, 2022
- early bird registration starting :
July 1rst July 24, 2022
More information on
https://jacc2022.sciencesconf.org
With as Plenary and Keynote speakers :
- Melanie Schnell
- Tiziana Benincori
- Marie-Claire Schanne Klein
- Hiroshi Yamamoto
- Eric Meggers
- Etienne Brasselet
- Mathieu Raynal
- Benjamin Abécassis
- Jess Wade
- Félix Freire

Laboratory: ICMCB
Kick-off meeting of ANR-CHIMERA
2022-05-10
CHIMERA standing for Chiral Induction from Microns to Electrons for Radiative Anisotropy, also in collaboration with Yann Battie (LCP-A2MP-Université de Lorraine/Metz)

Laboratory: IECB/ISM/CELIA/CBMN
Upcoming virtual event for Chirality.
2022-02-17
the
First Virtual Symposium on Chirality hosted by our very own Associate Editor, Prof. Oliver Trapp. The virtual symposium will take place on
Tuesday, 22 February 2022 from
12:00 PM UTC to
2:30 PM UTC
Register now to attend for free!
We welcome three
Chirality authors:
- Prof. Tamaki Nakano (Institute for Catalysis (ICAT), Hokkaido University, Japan) Light-induced Conformational Transition of Polymers and Small Molecules
Based on his work: Photo racemization of 2,2′-dihydroxy-1,1′-binaphthyl derivatives --- Chirality, 2021. https://doi.org/10.1002/chir.23400
- Prof. Dr. Alexander Kuhn (Groupe Nanosystèmes Analytiques, Université de Bordeaux, France) Unconventional Electrochemical Approaches for the Direct Readout of Chiral Information
Based on his work: Hybrid light-emitting devices for the straightforward readout of chiral information--Chirality, 2021. https://doi.org/10.1002/chir.23370
- Dr. Peter Wipf (Department of Chemistry, University of Pittsburgh, USA)
Enantioselective Imine Additions in the Preparation of Bioactive Lead Compounds Based on his work: Enantioselective synthesis and selective functionalization of 4-aminotetrahydroquinolines as novel glp-1 secretagogues --Chirality, 2021. https://doi.org/10.1002/chir.23403
More information on our speakers and the agenda for the event can be found on our
event website.
Laboratory: Chirality journal
PhotoElectron ELiptical Dichroism : PEELD
2022-02-09
The resonance-enhanced multiphoton ionization of chiral molecules by elliptically polarized laser pulses produces photoelectron angular distributions that are forward/backward asymmetric with respect to the light propagation axis. We investigate this photoelectron elliptical dichroism in the (2 + 1)-photon ionization of fenchone molecules, using wavelength tunable femtosecond UV pulses. We show that the photoelectron elliptical asymmetry is extremely sensitive to the intermediate resonant states involved in the ionization process, and enables electronic couplings to be revealed that do not show up so clearly when using circularly polarized light.
https://hal-cnrs.archives-ouvertes.fr/hal-03547281

Laboratory: CELIA
Revealing the Influence of Molecular Chirality on Tunnel-Ionization Dynamics
2021-12-21
The tunneling of a particle through a barrier is one of the most fascinating quantum phenomena. The motion taking place under the barrier, in a region forbidden by classical mechanics, is the subject of intense debate. Many experiments aim at measuring the time taken by the particle to go through the tunnel. Here, we take a completely different direction, revealing the influence of the dynamics under the barrier on the motion of the outgoing particle. Our approach combines two key elements: the barrier is chiral—its structure cannot be superimposed on its mirror image—and it rotates in time. Specifically, our barrier holds the electrons inside a chiral molecule, set spinning by a photoionizing laser field whose polarization rotates. The electrons must pass through the spinning barrier of the molecule to escape.

Laboratory: CELIA
Optically Active CdSe/CdS Nanoplatelets Exhibiting Both Circular Dichroism and Circularly Polarized Luminescence
2021-11-30
In this paper, chiroptical 2D CdSe/CdS nanoplatelets (NPLs) are prepared by ligand exchange approach, exhibiting both circular dichroism (CD) and circularly polarized luminescence (CPL). Furthermore, the CD and CPL signals are easily tuned via the design of the CdS-island structuration of the shell and its thickness which are controlled with the reaction time.
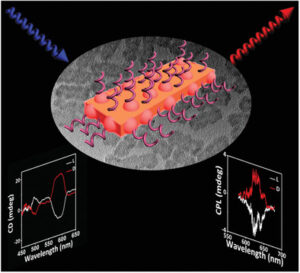
Laboratory: ICMCB
Ultrafast relaxation investigated by photoelectron circular dichroism: an isomeric comparison of camphor and fenchone
2021-11-26
Circular dichroism in the photoelectron angular distribution decays exponentially in ∼730 fs from a +9% forward amplitude during the first hundreds of femtoseconds to reach an asymptotic −2% backward amplitude after to have photoexcited at ~6 eV. This time-scale is drastically shorter than in fenchone, its isomer. Our analysis allows us to evaluate the impact of the anisotropy of excitation and reveal a breakdown of the Franck-Condon approximation.

Laboratory: CELIA
Interdisciplinarity and Chirality
2021-10-25
Symmetry and Chirality: Where Physics Shakes Hands with Chemistry and Biology by Laurence Barron

Laboratory: a review article


 DOI: 10.1039/D3CC04391F
DOI: 10.1039/D3CC04391F




















